測試報告
由於測試報告眾多,未能盡錄,如有查詢請聯絡我們。
產品認證
各國醫療衛生局認證
選擇地區 Select Your Location
您正在瀏覽 You are currently viewing: 香港 Hong Kong|繁體中文
請選擇地區 Please select your location:
由於測試報告眾多,未能盡錄,如有查詢請聯絡我們。

 2+
2+NIOSH 42 CFR 84 N95 (US) Filtration and Breathing Resistance Test

 7+
7+ASTM F2100-19 Level 3 (US) standards/
EN14683:2019 Type IIR (EU) standards/
VFE ≥99.9%

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices
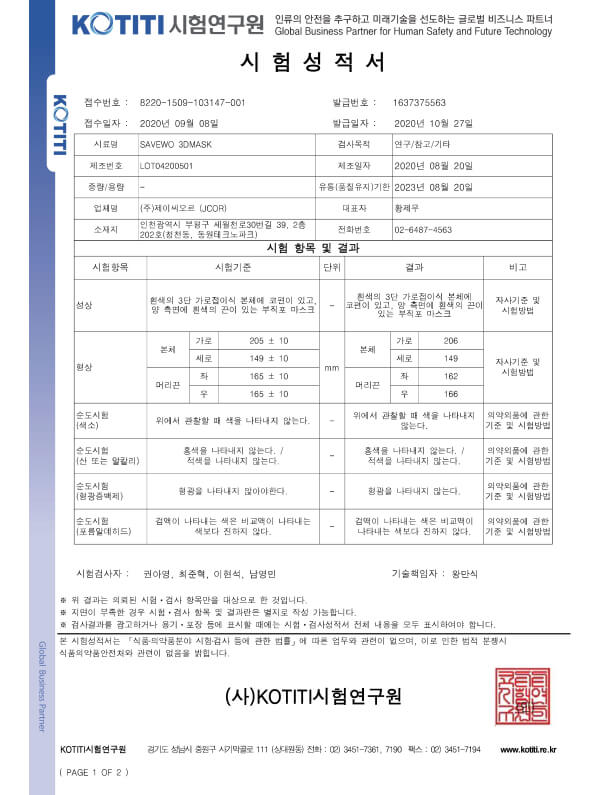
 3+
3+KMOEL-2017-64 KF94 (Korea) standard

 2+
2+NIOSH 42 CFR 84 N95 (US) Filtration and Breathing Resistance Test

 7+
7+ASTM F2100-19 Level 3 (US) standards/
EN14683:2019 Type IIR (EU) standards/
VFE ≥99.9%

 2+
2+ASTM F2100-20 Level 3 (US) / EN14683:2019 Type IIR (EU) - Accelerated Aging Test as per ASTM F1980-16 (3 years)

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

 2+
2+NIOSH 42 CFR 84 N95 (US) Filtration and Breathing Resistance Test

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices
-EN14683-2019-TypeIIR(EU).jpg)
 6+
6+ASTM F2100-19 Level 3 (US) standards/
EN14683:2019 Type IIR (EU) standards
-ClassII-medical-device-510(k).jpg)
 4+
4+FDA (US) Class II medical device 510(k)

 6+
6+ASTM F2100-19 Level 3 (US) standards


TYPECOOL+ technology

 4+
4+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

 2+
2+TYPECOOL+ / TYPECOOL+ EX technology

 2+
2+ISO10993-5 MEM Elution Test, Biocompatibility of medical devices

 8+
8+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices
