DEEP SEA BLUE CASTLE GREY FOREST GREEN DARK SOUL BLACK



E
W
C
O
L
O
R NEW COLOR


The Savewo Ultra-Seal Technology is used on the 3DMASK to prevent the exposure of the white internal layer. The perfect seal gives it the perfect appearance.


- Color Masterbatch Technology with highest safety level
- Not containing 212 SVHC high concern materials
- Not containing Azo Dye in Textile
- Not containing fluorescein, latex-free and antiallergic

UltraSpunBond is a super water-repellent fabric that provides good splash protection and is capable of resisting droplets and splashes at a pressure of 160 mmHg.

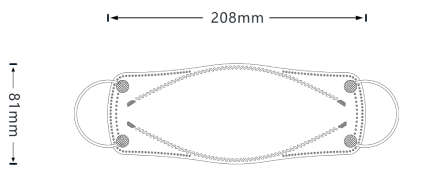
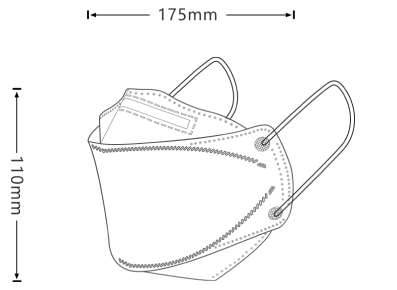
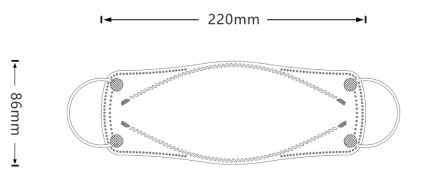
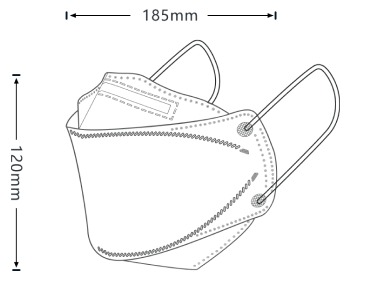
Our patented 3D design fits better to your nose bridge and your face shape in order to prevent not only inward leakage but also fogging for glasses wearers.
WIth the UltraSeal welding technology, it creates a firmer and more durable three-dimensional structure for our thin breathable masks.

ESPP with a smooth and hydrophilic inner layer can maintain consistent humidity under the mask either in a wet or dehydrated condition.

- Bacterial Filtration Efficiency (BFE) ≥99.9%
- Particle Filtration Efficiency (PFE) ≥99.9%
- Viral Filtration Efficiency (VFE) ≥99.9%
- Fluid Resistance: Resists Synthetic Blood @ 160mmHg of pressure
- Filtration is effective for 24 hours+
- Filtration efficiency maintained 99.9% upon exposure to 100% relative humidity for 24 hours
Filtration Efficiency ≥99.9%






SAVEWO Products have Passed Multiple Tests of International Standards!
- Bacterial Filtration Efficiency BFE≥99.9%
- Particle Filtration Efficiency PFE≥99.9%
- Viral Filtration Efficiency VFE≥99.9%
- Innovative
filtration technology with ultra-high breathability and effective for 24 hours
- Ultra-low Breathing Resistance < 2.4mmH2O
- Fluid Resistance: Resists Synthetic Blood @ 160mmHg of pressure
- Smooth and hydrophilic ESPP inner layer, skin friendly material
- Conforms with KMOEL-2017-64 KF94 (Korea) respirator standards
- Conforms with GB2626-2019 KN95 (CN) respirator standards
- Passed NIOSH 42 CFR 84 N95 (US) filtration and breathing resistance test
- Conforms with ASTM F2100-19 Level 3 (US) standards
- Conforms with EN14683:2019 Type IIR (EU) standards
- Conforms with JIS T 9001:2021 Class III (Japan) standards
- Passed ISO10993-5 MEM Elution Test and ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices
- Passed the Microbial Cleanliness (Bioburden) Test
- Color Masterbatch Technology with highest safety level
- Not containing 212 SVHC high concern materials
- Not containing Azo Dye in Textile
- Not containing fluorescein, latex-free and antiallergic
- ISO9001:2015 Quality Management System
- ISO13485:2016 Medical Devices Quality Management System
- ISO14001:2015 Environmental Management System
- ISO14644-1:2015 Class 7 Cleanroom
- CE Marking Certification (EU)
- Regulated by FDA (US), Registration Number: 3017178899
- Regulated by MFDS (KFDA) (Korea), Registration Number: 202107925
- Designed and Made in Hong Kong, individual packing