CERTIFICATES & TESTING REPORTS
These are only parts of our reports, please do not hesitate to contact us for any enquiries.
Product Certification
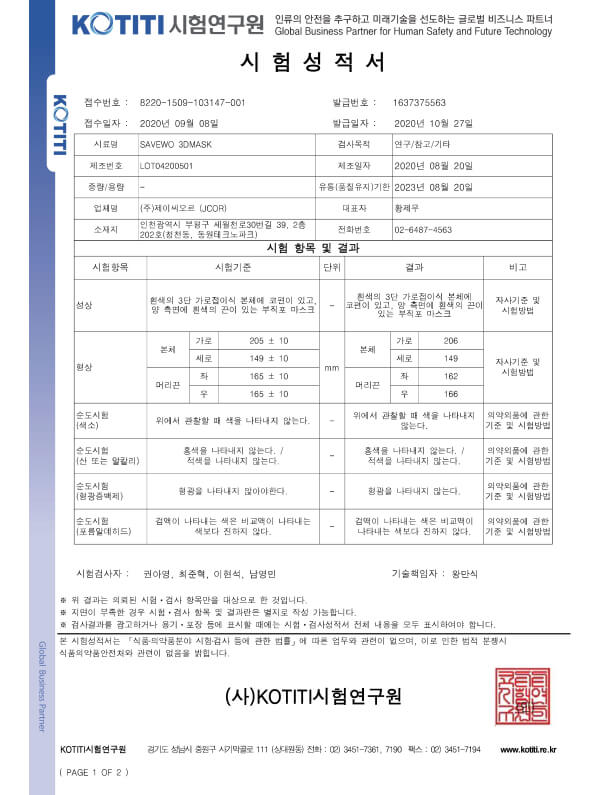
KMOEL-2017-64 KF94 (Korea) standard

GB2626-2019 KN95 (CN) standard

 2+
2+NIOSH 42 CFR 84 N95 (US) Filtration and Breathing Resistance Test

 7+
7+ASTM F2100-19 Level 3 (US) standards/
EN14683:2019 Type IIR (EU) standards/
VFE ≥99.9%
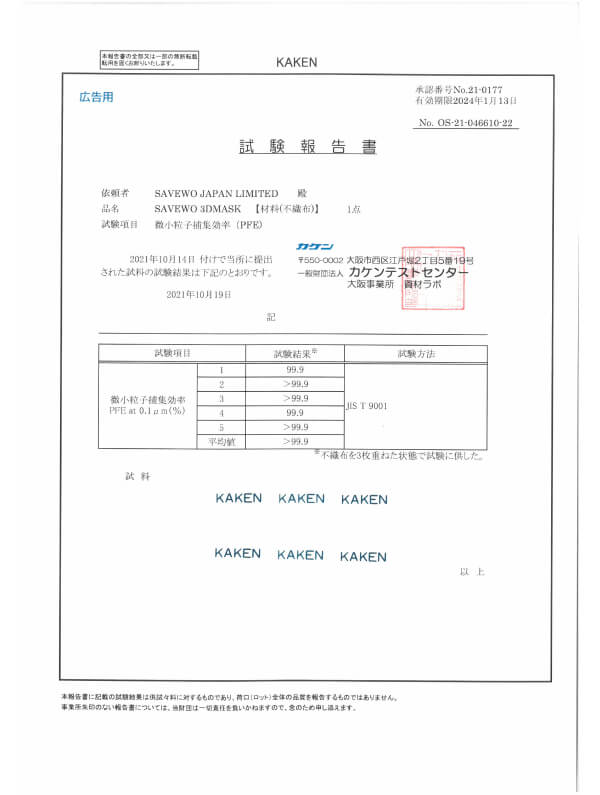
JIS T 9001:2021 Class III, BFF/PFE/VFE and more.. Japanese medical face masks standard (by KAKEN)

ISO14362-1 2017 Azo Dyes Test

ISO10993-5 MEM Elution Test, Biocompatibility of medical devices

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

TYPECOOL+ technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.
.jpg)
3DMASK KIDS
KMOEL-2017-64 KF94 (Korea) standard

3DMASK KIDS
ASTM F2100-19 Level 3 (US) standards

3DMASK KIDS
EN149:2001+A1:2009
Breathing Resistance

3DMASK KIDS
EN14683:2019 Type IIR (EU) standards

Certificate of Free Sales, Korea

EN149:2001 + A1:2009 FFP2 (EU) Respirator Standards

 3+
3+KMOEL-2017-64 KF94 (Korea) standard

GB2626-2019 KN95 (CN) standard

 2+
2+NIOSH 42 CFR 84 N95 (US) Filtration and Breathing Resistance Test

 7+
7+ASTM F2100-19 Level 3 (US) standards/
EN14683:2019 Type IIR (EU) standards/
VFE ≥99.9%

 2+
2+ASTM F2100-20 Level 3 (US) / EN14683:2019 Type IIR (EU) - Accelerated Aging Test as per ASTM F1980-16 (3 years)

COVID-19 Medical Device Class I, Health Canada

ISO10993-5 MEM Elution Test, Biocompatibility of medical devices

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

TYPECOOL+ technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.

Certificate of Free Sales, Korea

EU Declaration of Comformity
-SL52105232542501TX.jpg)
EN149:2001 + A1:2009 FFP3 (EU) Respirator Standards
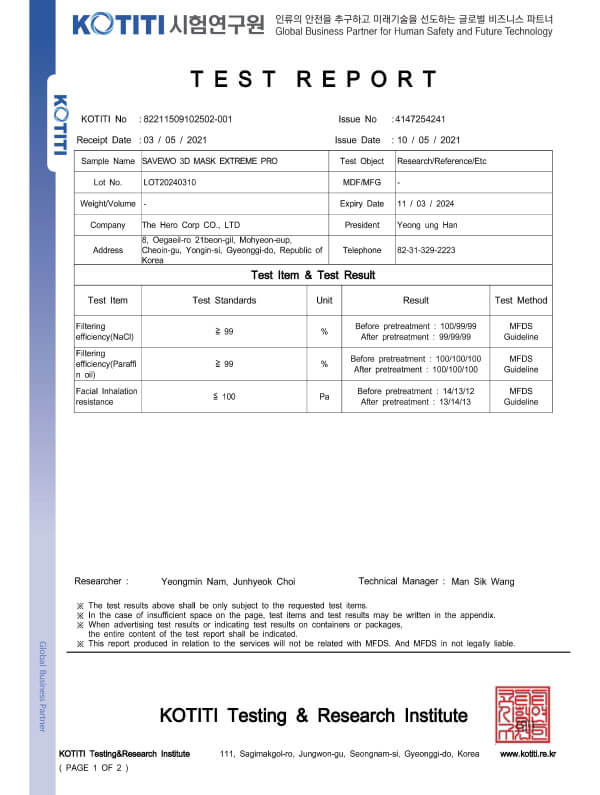
KMOEL-2017-64 KF99 (Korea) Respirator Standards

ASTM F2100-19 Level 3 (US) standards/

EN14683:2019 Type IIR (EU) standards

COVID-19 Medical Device Class I, Health Canada

VFE at an Increased Challenge Level 99.99%

ISO14362-1:2017 Azo Dyes Test

ISO10993-5 MEM Elution Test, Biocompatibility of medical devices

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

TYPECOOL+EX technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.

EN149:2001 + A1:2009 FFP2 (EU) Respirator standards
.jpg)
KMOEL-2017-64 KF94 (Korea) standard

 2+
2+NIOSH 42 CFR 84 N95 (US) Filtration and Breathing Resistance Test

ASTM F2100-19 Level 3 (US) Standards

EN14683:2019 Type IIR (EU) Standards

ISO10993-5 MEM Elution Test, Biocompatibility of medical devices

 2+
2+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

TYPECOOL+ technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.

ASTM F2100-19 Level 3 (US) standards

EN14683:2019 Type IIR (EU) standards

COVID-19 Medical Device Class I, Health Canada
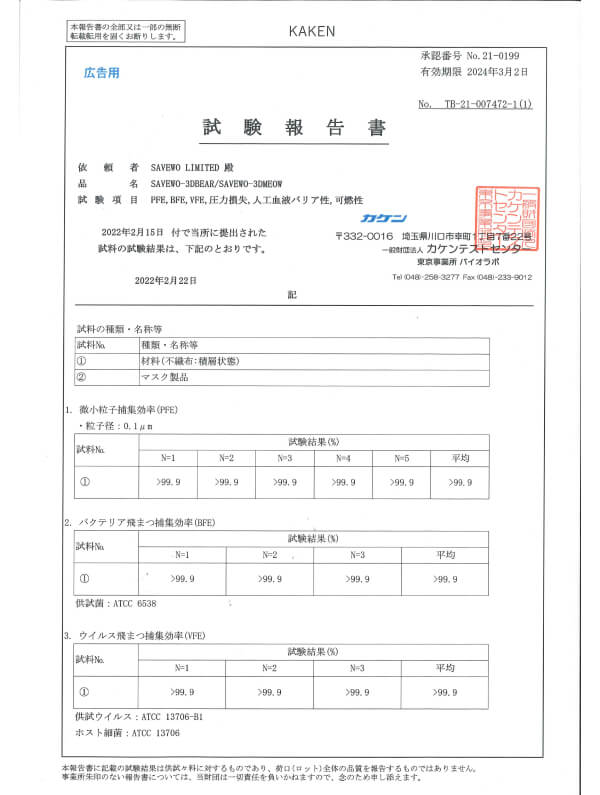
JIS T 9001:2021 Class III, BFF/PFE/VFE and more.. Japanese medical face masks standard (by KAKEN)

TYPECOOL+ technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.
.jpg)
KMOEL-2017-64 KF94 (Korea) standard

ASTM F2100-19 Level 3 (US) standards

EN14683:2019 Type IIR (EU) standards

JIS T 9001:2021 Class III, BFF/PFE/VFE and more.. Japanese medical face masks standard (by KAKEN)

TYPECOOL+ technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.

ASTM F2100-19 Level 3 (US) standards

EN14683:2019 Type IIR (EU) standards

TYPECOOL+ technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.
-ClassII-medical-device-510(k).jpg)
 4+
4+FDA (US) Class II medical device 510(k)

 6+
6+ASTM F2100-19 Level 3 (US) standards

EN14683:2019 Type IIR (EU) standards

TYPECOOL+ technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.

ISO10993-5 MEM Elution Test, Biocompatibility of medical devices

 4+
4+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices

SARS-CoV-2 Test
EN14476:2019 (with modification), 3g salt/ 300ml water, mode 2 (10s)

General Purpose
EN1276:2019, 3g salt/ 300ml water, mode 2 (10s)

Hand Hygiene
EN1276:2019, 3g salt/ 300ml water, mode 2 (10s)

Hypochlorite content, 1g salt/ 300ml water, mode 1-2 (3-5mins)

Active Chlorine Content, 0-30 days

Hypochlorite content, tap water, 3g salt/ 300ml water, mode 1-2 (3-5mins)

XRF Screening and Chemical Confirmation Test Report

EMC Directive 2014/30/EU (IEC)
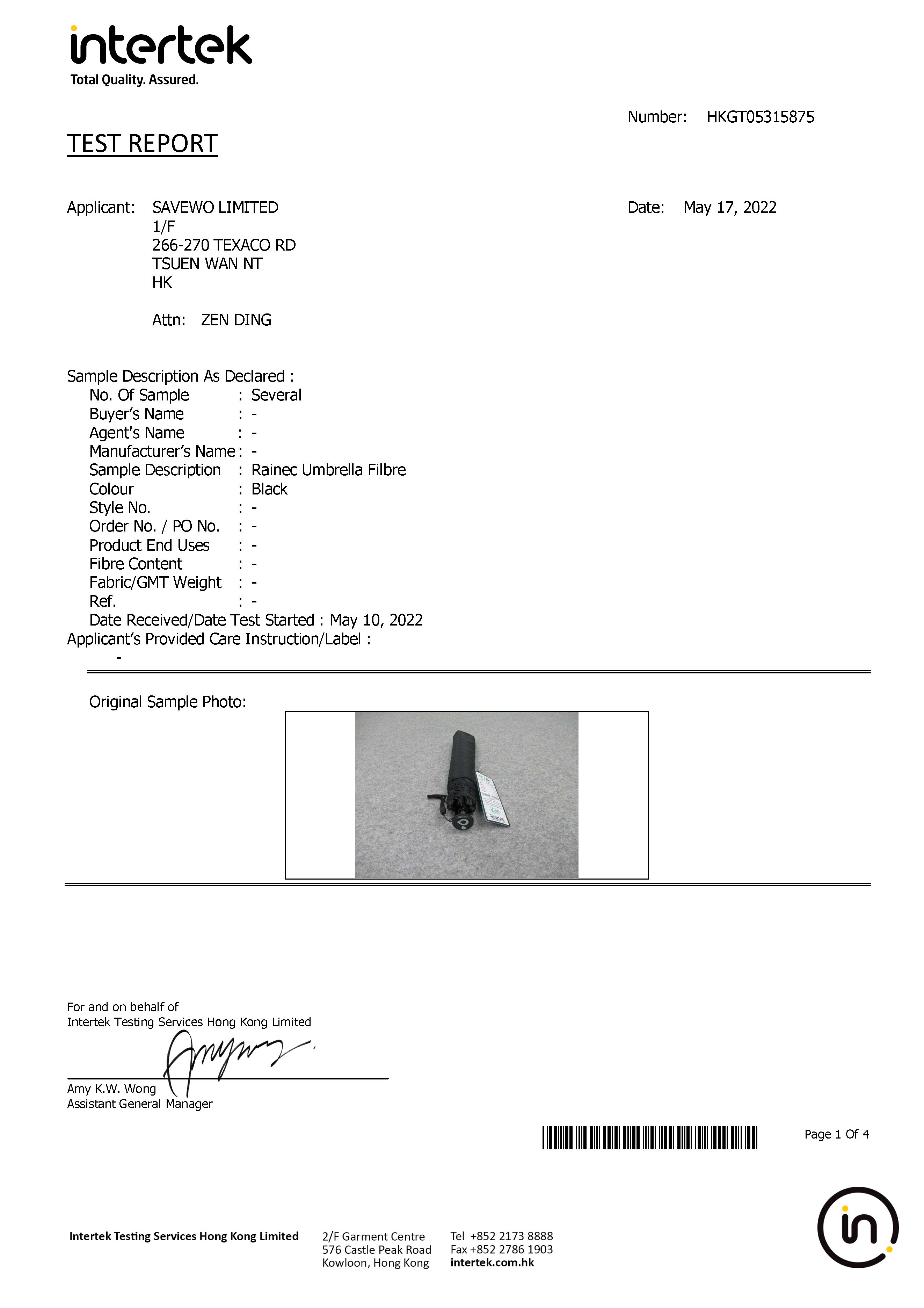
UVR Transmission of Dry Textile, Light Blocking, Spray Test
Factory Certification

ISO 9001:2015 Quality Management System

ISO 13485:2016 Medical Devices Quality Management System

ISO 14001:2015 Environmental Management System

ISO 14644-1:2015 Class7 Cleanroom

Facility and Merchandise Authorization (FAMA) (By Disney)

Factory Registration
Material Certification

 2+
2+TYPECOOL+ / TYPECOOL+ EX technology
*As proven by professional laboratory tests, the filtration efficiency is maintained at an astonishing 99.9% upon exposure to 100% relative humidity for 24 hours.

ISO14362-1 2017 Azo Dyes Test

 2+
2+ISO10993-5 MEM Elution Test, Biocompatibility of medical devices

 8+
8+ISO10993-10 Skin Irritation and Sensitization Test, Biocompatibility of medical devices
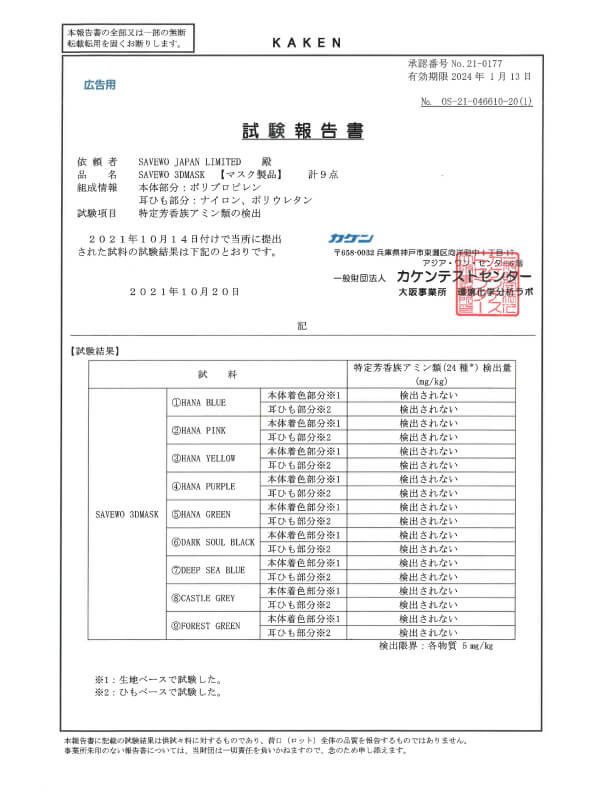
JIS T 9001:2021 Class III
特定芳香族アミンの検出
Japanese medical face masks standard (by KAKEN)
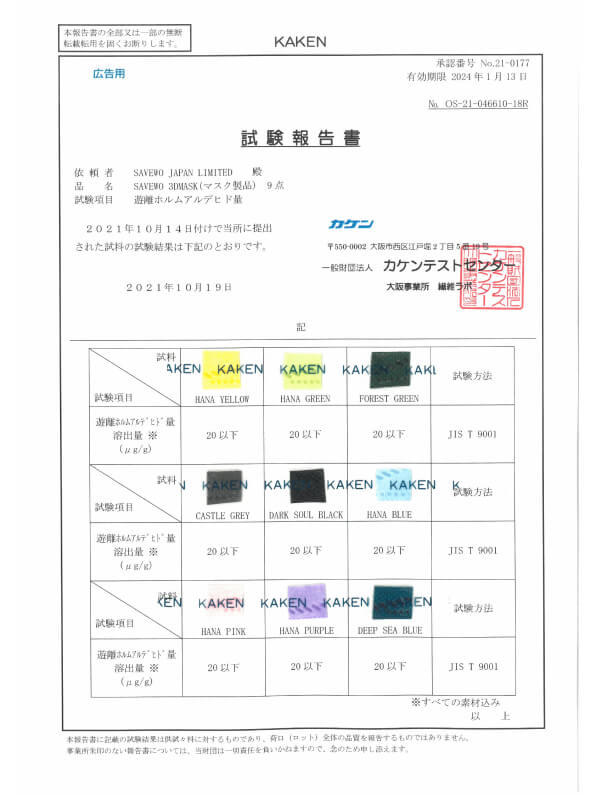
JIS T 9001:2021 Class III
遊離ホルムアルデヒド量
Japanese medical face masks standard (by KAKEN)
Certification - Medical and Health Bureau
.jpg?id=1)
 2+
2+CE Marking Certification (EU)
-ClassII-medical-device-510(k).jpg)
 4+
4+FDA (US) Class II medical device 510(k)

Regulated by FDA (US)
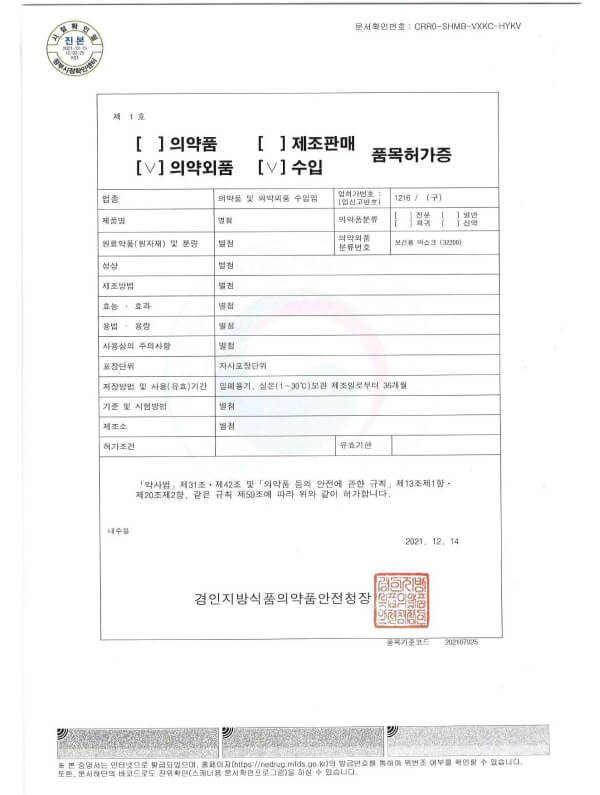
Regulated by MFDS (KFDA) (Korea)

 3+
3+COVID-19 Medical Device Class I, Health Canada
-Module-B.jpg)
Module B: EU Type Examination / EN149:2001+A1:2009
FFP3 respirator standards
-CERTIFICATE.jpg)
Module B: EU Type Examination / EN149:2001+A1:2009
FFP2 respirator standards
2016-425-Module-D.jpg)
Regulation (EU) 2016/425 Module D FFP2 & FFP3 Protective Respiration
